Portfolio
How this looks in practice
Much of the work I do doesn't end with a rewritten product page. It ends with a founder or marketing team who understands exactly where their messaging stands, what the risk actually is, and what their options are.
The examples below show two pre-launch brands I worked with on claims alignment and positioning strategy. In both cases, as with most supplement brands at this stage, the early language created regulatory risk that wasn't immediately obvious. The work was to identify where that risk sat, reframe the messaging to sit within compliant guardrails, and leave them with something they could use consistently going forward.
Brand names have been anonymised, but the work shown is real.
Caffeine support supplement: pre-launch messaging review
A pre-launch supplement brand targeting consumers who enjoy caffeine but are sensitive to its effects. The founder had a strong concept and clear brand voice. The difficulty was in how that idea showed up in the language: early messaging framed the product too closely as a solution to caffeine side effects, creating implied symptom-relief risk under UK CAP/ASA guidance.
The work involved reviewing proposed taglines, educational content, and formulation-level claim eligibility, then restructuring the positioning so it could be supported by authorised UK health claims without losing the brand's original feel.
High-risk language was removed or reframed before launch. Educational content was retained for SEO value but separated from product references to remove implied treatment associations.
The founder left with a messaging foundation she could use consistently across ads, packaging, and content, without second-guessing the wording every time.
"I came to Victoria feeling completely overwhelmed about what I could and couldn't say. She helped me understand exactly which claims were allowed, which carried risk, and how to reframe the language so it stayed compliant without losing clarity or appeal." — Founder
Children's supplement brand: pre-launch messaging review
A children's supplement brand built around nutritional products and family rituals, using emotionally resonant language centred on calm, confidence, and bravery. The challenge was navigating how that emotional positioning showed up in product messaging, particularly where language risked implying emotional or therapeutic outcomes in a children's context.
The primary risk wasn't isolated wording. It was how emotional language, rituals, and products were being read together across different channels.
The work involved separating sales-intent assets from non-sales contexts, restructuring product copy to lead with nutrition rather than emotional outcomes, and putting creator and social guidance in place so the brand's emotional depth could be retained without creating implied claims.
The result was a clear, repeatable framework the founder could apply consistently across future content and campaigns.


Compliance-Informed Copywriting That Protects, Persuades, and Performs
Compliance copywriting involves more than just choosing the right words to reduce risk across highly regulated platforms. Your messaging still needs to connect with your audience.
These examples demonstrate how I audit, rewrite, and create content designed to:
Avoid unauthorised health claims
Reduce the risk of listing rejections and suspensions
Build trust with both customers and platforms
Keep your products visible and selling
Note: These samples are based on mock scenarios and anonymised examples.
Sample 1: Product Description Rewrite + Audit Snapshot
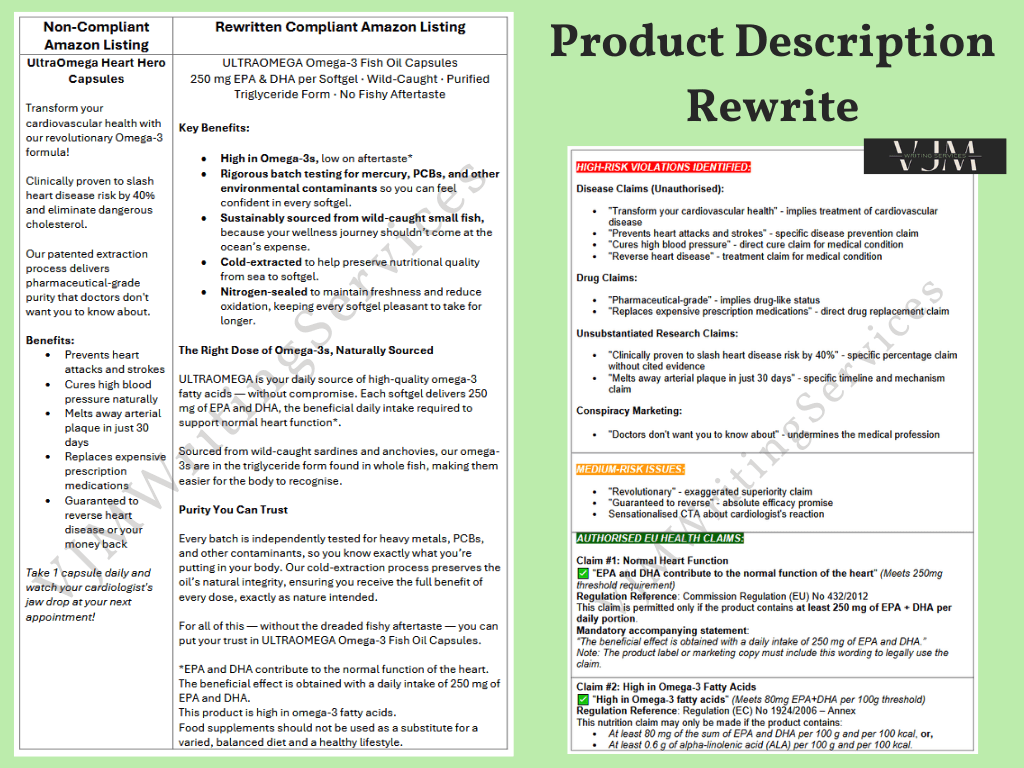
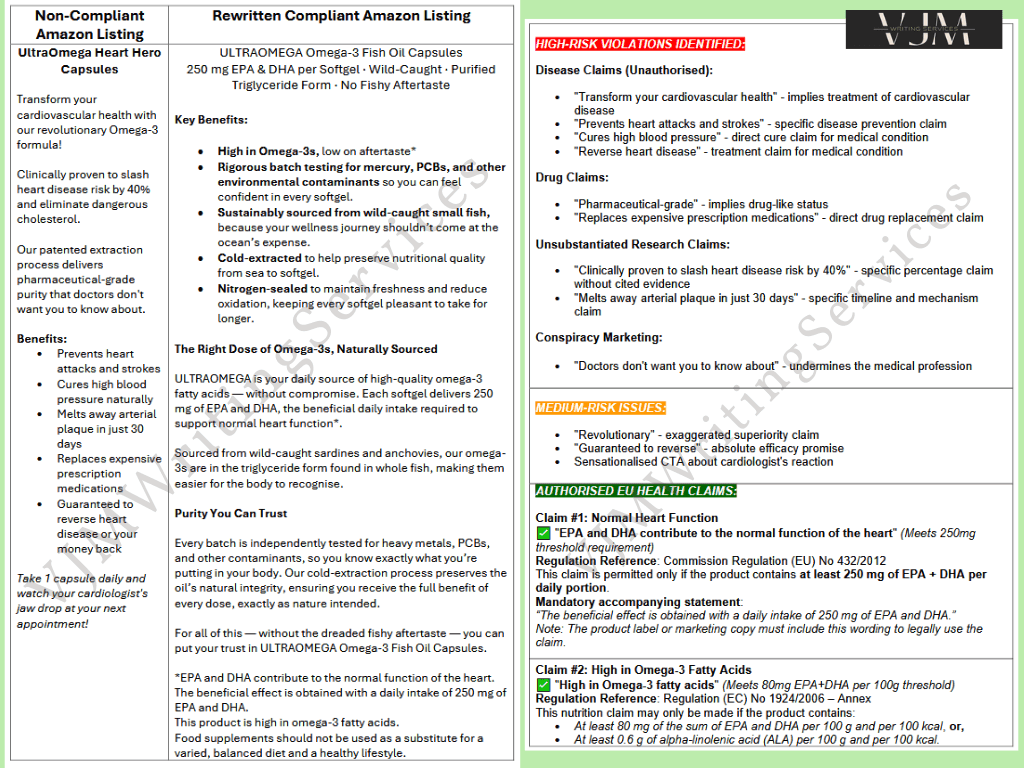
This example shows how I rewrote a flagged Amazon listing for ULTRAOMEGA Heart Hero Capsules. The original product description contained multiple high-risk violations, including unauthorised disease claims, unsubstantiated research claims, and banned drug references. These triggered platform rejections and posed a significant regulatory compliance risk.


Audit Snapshot
High-Risk Violations Identified include:
Unauthorised disease claims: “Prevents heart attacks,” “Cures high blood pressure,” “Reverses heart disease”
Unsubstantiated clinical claims: “Clinically proven to slash heart disease risk by 40%”
Drug-related claims: “Pharmaceutical-grade purity” and “Replaces prescription medications”
Conspiracy marketing: “Doctors don’t want you to know about this”
Approved Claims Integrated:
EPA and DHA contribute to the normal function of the heart” (Commission Regulation (EU) No 432/2012)
High in Omega-3 fatty acids” (Regulation (EC) No 1924/2006)
Outcome
The rewritten listing:
Removed all high-risk language
Incorporated authorised EU health claims
Added required disclaimers and compliant phrasing
Optimised content to reduce Amazon/Google rejection risks and build consumer trust
Sample 2: Article Rewrite + Compliance Audit Snapshot
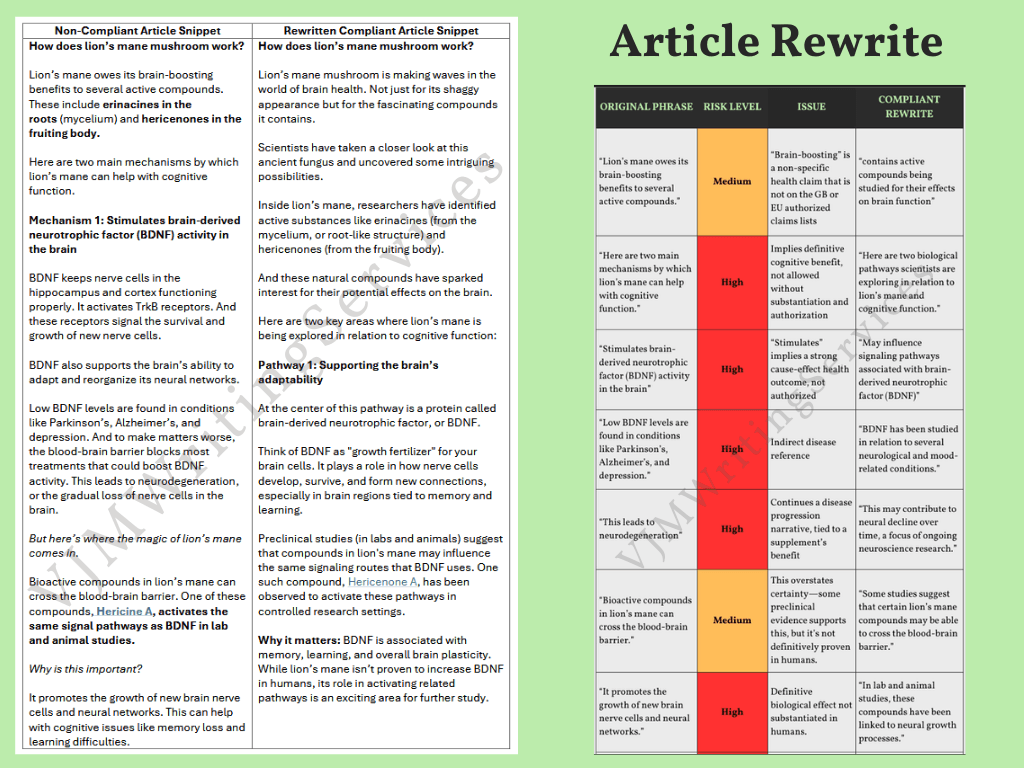
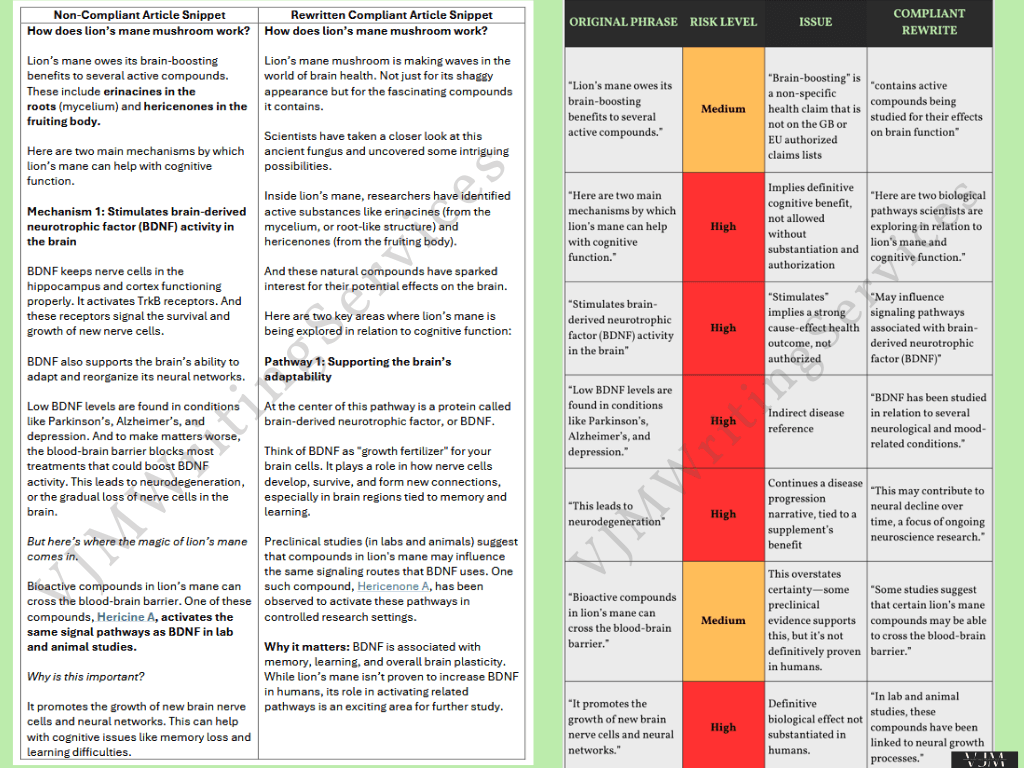
This example shows how I rewrote a blog article on lion’s mane mushrooms that contained multiple high-risk compliance issues. The original copy overstated scientific findings, included unauthorised cognitive health claims, and referenced diseases in a way likely to trigger ASA/EFSA scrutiny.
The goal was to rewrite the article so it remained educational, SEO-friendly, and engaging, while removing unsupported claims and integrating safer, compliance-informed phrasing.
Audit Snapshot
High-Risk Violations Identified:
“Brain-boosting benefits” → Non-specific health claim, not on EU/UK authorised claims list
“Helps with cognitive function” → Implies treatment/prevention of disease, not authorised
References to Parkinson’s, Alzheimer’s, and depression → High-risk indirect disease claims
“Promotes growth of new brain cells” → Definitive biological outcome not substantiated in humans
Action Taken:
Reframed findings using evidence-informed, compliant phrasing
Removed direct disease references and overstated claims
Retained SEO value by using audience-relevant keywords like “brain health” and “adaptability”
Added clear context about preclinical research limitations
Outcome
The rewritten article is:
Compliant with ASA/EFSA expectations
Educational and engaging while avoiding overpromises
Structured for long-term SEO performance
Built to reduce platform and regulatory risk
Join My Email List
Stay connected and get the latest tips and insights on creating effective content for your nutrition and wellness brand. From content strategy advice to practical writing tips, I’ll share valuable insights straight to your inbox.
The information and services provided by VJM Writing Services are for educational and marketing purposes only and do not constitute legal or regulatory advice.
While we create content designed to align with regulatory frameworks (ASA, EFSA, FDA, FTC, etc.), compliance involves many factors beyond marketing copy, including formulations, labelling, and internal processes.
We cannot guarantee product approvals or prevent regulatory action, and we recommend consulting a qualified legal or regulatory professional for full compliance support.
© vjmwritingservices 2025

